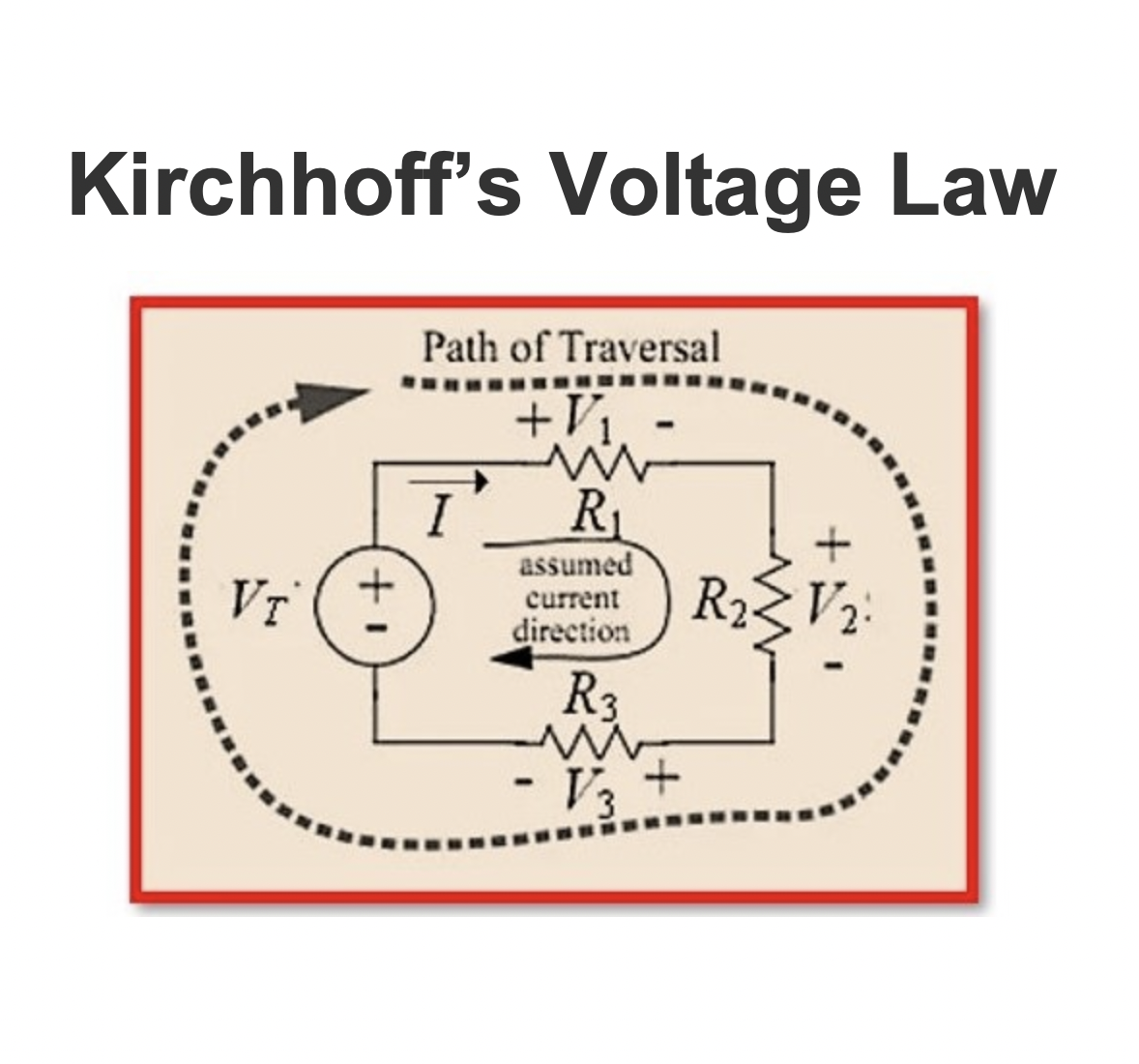
Kirchhoff’s Voltage Law
Kirchhoff’s Laws include two fundamental principles in electrical circuit analysis: Kirchhoff’s Current Law (KCL) (Kirchhoff’s First Law or Kirchhoff’s 1st Law)& Kirchhoff’s Voltage Law (KVL) (Kirchhoff’s Second Law or Kirchhoff’s 2nd Law).These principles serve as essential tools for evaluating complicated electrical circuits, allowing engineers &researchers to predict &comprehend the behavior of circuits in various configurations. Kirchhoff’s Laws are widely applied in Electronics
Rabert T
03/10/2024
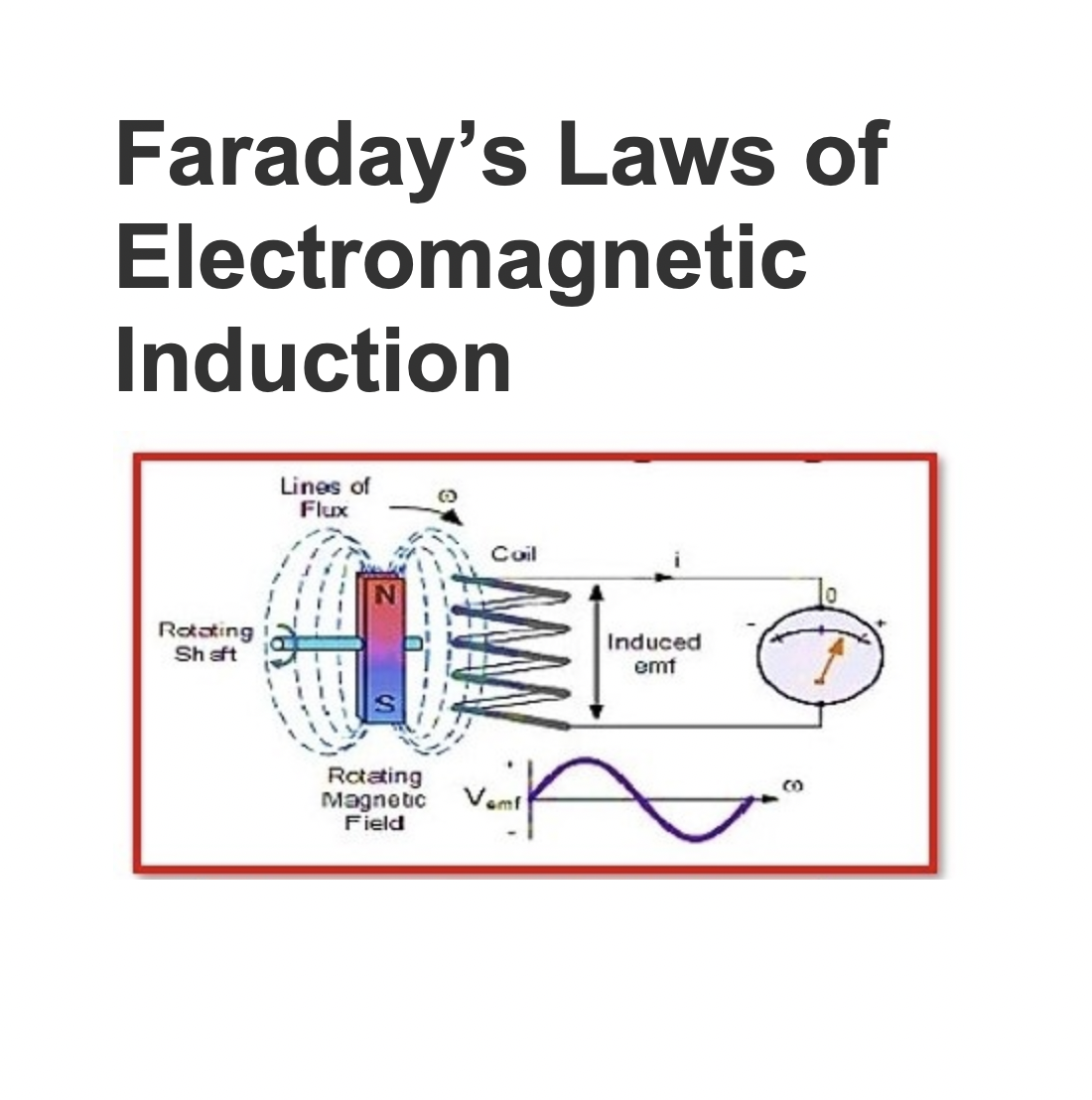
Faraday’s Laws of Electromagnetic Induction
Faraday’s law, also known as Faraday’s law of electromagnetic induction, is a fundamental law of electromagnetism that predicts how a magnetic field interacts with an electric circuit to produce an electromotive force (EMF). This is referred to as “electromagnetic induction.”Faraday laws of electromagnetic induction:Faraday’s Laws of Electromagnetic Induction consist of two laws:1. The first law describes the induction of emf in a conductor and2. The second law calculates the conductor’s generat
Rabert T
03/10/2024

Statement, Formula, Uses, and Limits of Coulomb’s Law
Coulomb’s Law:Coulomb’s law states that the force of attraction or repulsion between two chargesis directly proportional to the product of their charges and inversely proportional to the square of their distance from one another. It functions on the section that connects the two charges that are regarded of as point charges.Coulomb’s Law Formula:Where,F= Electric Force,K= Coulomb’s Constant,q1, q2= chargesr= between distanceWhat Is one Coulomb of Charge?It is known as a coulomb when two charges
Rabert T
03/10/2024
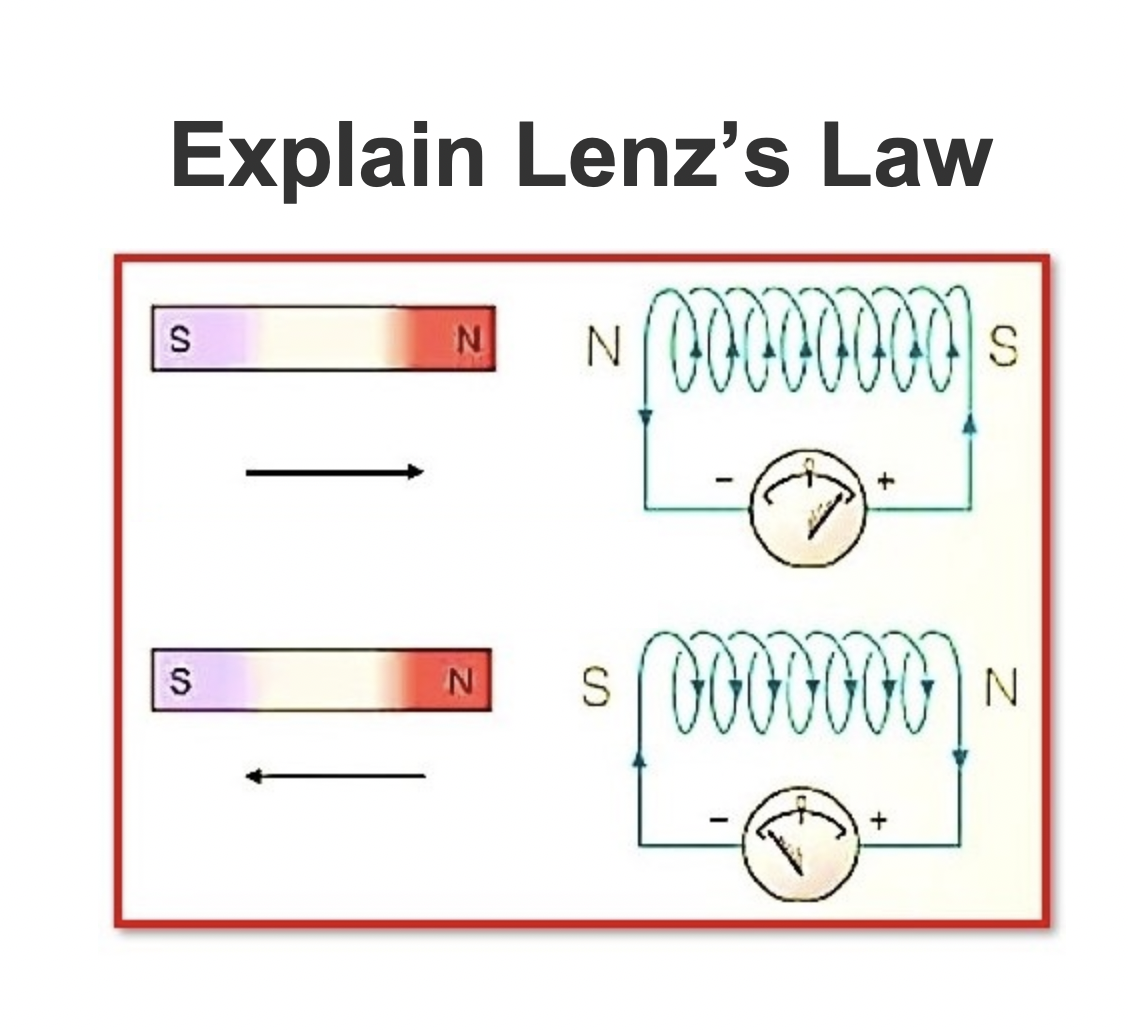
Explain Lenz’s Law
What is Lenz’s Law?Lenz’s law of electromagnetic induction explains that the direction of the current induced in a conductor by a changing magnetic field (as per Faraday’s law of electromagnetic induction) is such that the magnetic field created by the induced current opposes the changing magnetic field that caused it. The direction of thecurrent flow is shown byFleming’s right-hand rule.Lenz’s law is based on Faraday’s law of induction, which says that a changing magnetic field will cause a cur
Rabert T
03/10/2024
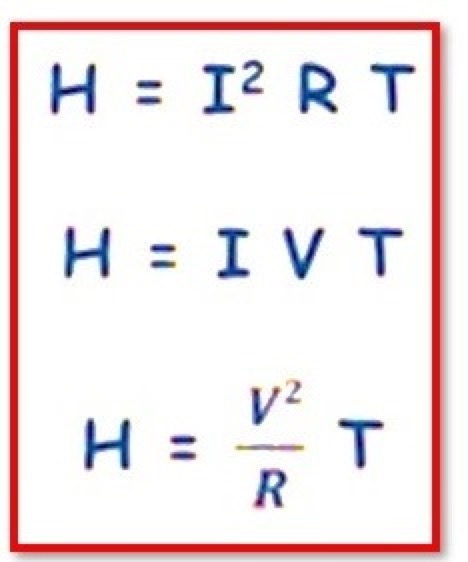
State Joule’s Law
Joule’s Law:According to Joule’s Law, when a current flows in a conductor, the amount of heat generated is proportional to the current, resistance, and time in the current flowing.Joule’s law of Heating:The unit of Joules is used to measure the amount of heat generated by the movement of current in an electric wire. Following is a description of how Joule’s law is represented mathematically and explained.When the electrical resistance of the wire and the time the current is flowing are constant,
Rabert T
03/09/2024
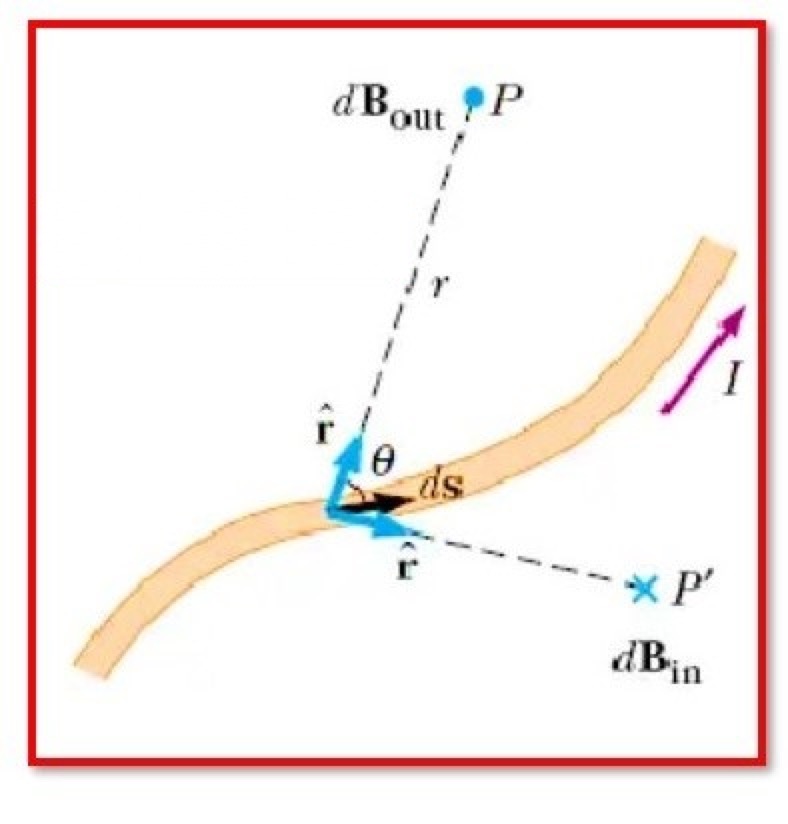
Explain Biot Savart Law
What exactly is Biot Savart Law?The Biot Savart Law is a mathematical equation that describes the magnetic field produced by a constant electric current. It connects the magnetic field to the electric current’s size, direction, length, and proximity. Ampere’s circuital law and Gauss’ theoremare both consistent with the Biot-Savart law.The Biot-Savart law is essential to magnetostatics, having a similar function toCoulomb’s lawin electrostatics.Biot Savart Law Statement:According to the Biot-Sava
Rabert T
03/09/2024
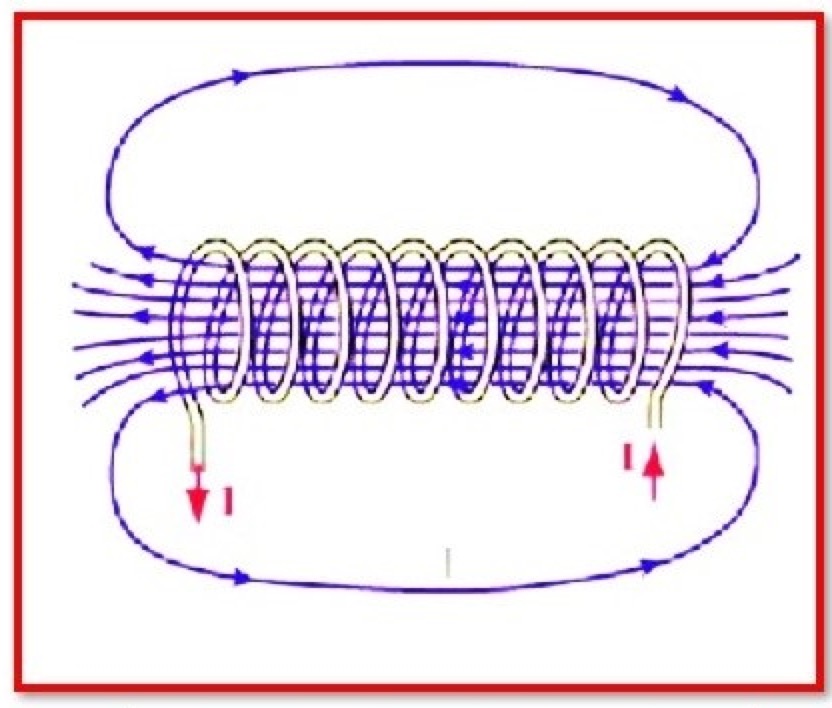
Ampere’s circuital law
Ampere’s circuital law is a fundamental law in electromagnetism that relates the magnetic field around a conductor to the electric current flowing through the conductor. It is named after French scientist André-Marie Ampère, who developed the law in the early 19th century.Ampere’s circuital law Equation:Ampere’s circuital law can be expressed mathematically as:∮B⋅ds =µ0Iencwhere:∮B⋅ds – The integral of the magnetic field (B) around a closed path (ds)µ0 – The permeability of free space, a constan
Rabert T
03/09/2024
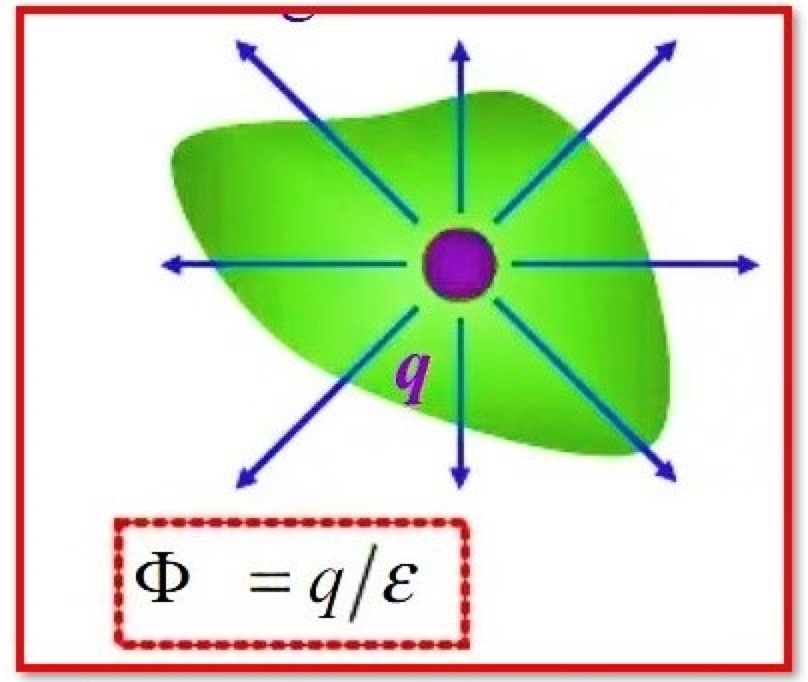
State Gauss’s law
In physics, Gauss’s Law is a fundamental relationship that connects the distribution of electric charge to the resulting electric field. It is a generalization of Coulomb’s Law, which describes the electric force between two-point charges. Gauss’s Law states that the flux of the electric field through any closed surface is equal to the charge enclosed within that surface.Gauss’s law Mathematical Expression:Mathematically, Gauss’s Law can be expressed as:∫E⋅dA = q/εwhere:E – The electric fielddA
Rabert T
03/09/2024
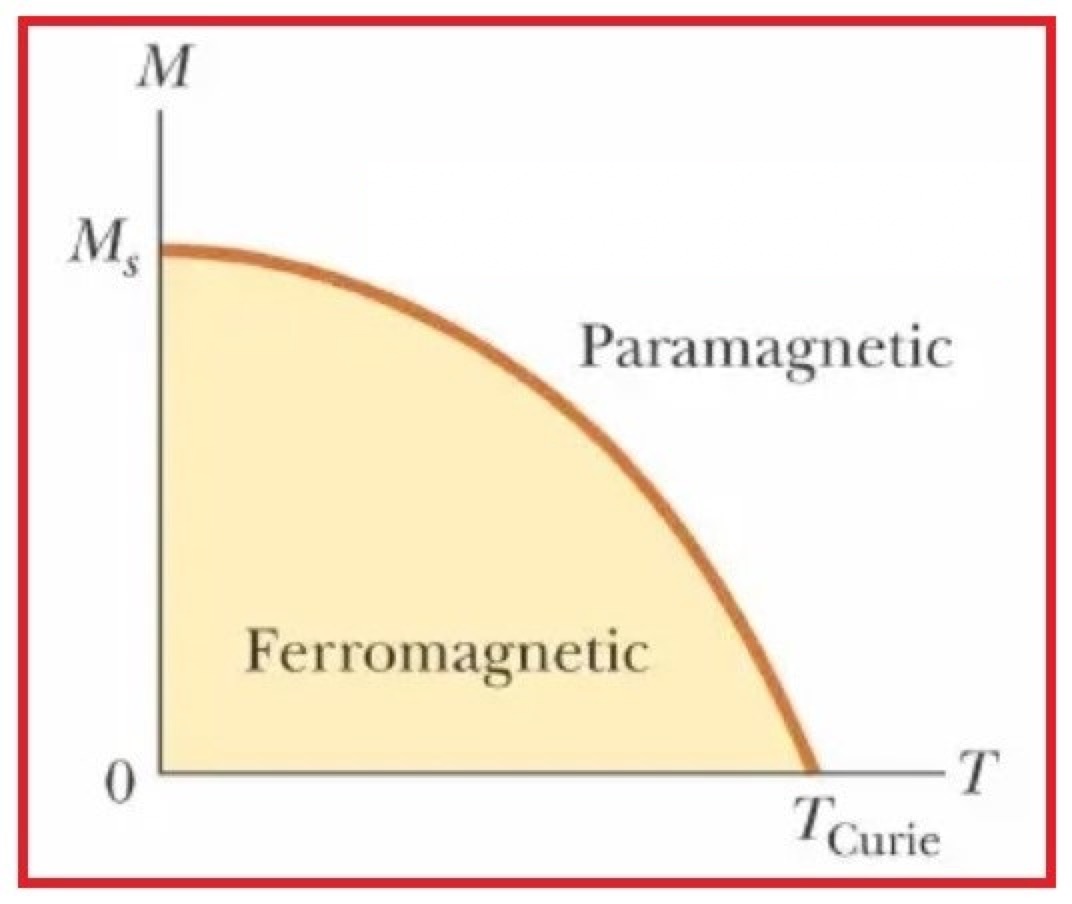
Curie’s Law
Curie’s Law is a relationship in physics that describes the behavior of magnetic materials at different temperatures. It states that the magnetic moment per unit volume of a material is directly proportional to the temperature. The magnetic moment of a material is a measure of the strength of its magnetization.Curie’s Law Expression:Mathematically, Curie’s Law can be expressed as:M/V = C/Twhere:M – The magnetic moment per unit volumeV – The volume of the materialC – A constant of proportionality
Rabert T
03/09/2024
Hopkinson’s Law
Hopkinson’s Law is a relationship in materials science that describes the behavior of materials under high strain rates. It states that the stress of a material is proportional to the strain rate at which it is deformed. Hopkinson’s Law is named after Sir Benjamin Baker Hopkinson, who first proposed it in the early 20th century.Hopkinson’s Law Mathematical Expression:Mathematically, Hopkinson’s Law can be expressed as:σ = k ε̇where:σ – The stress of the materialk – The material’s strength coeffi
Rabert T
03/09/2024









