What is SF6 Gas?
What is SF6 Gas?
SF6 Gas Definition
SF6 gas is defined as a compound of one sulfur atom and six fluorine atoms, known for its stability and use in electrical systems.
Manufacturing Process
SF6 gas is commercially manufactured by the reaction of fluorine (obtained by electrolysis) with sulfur.
During process of producing of this gas, other byproducts like SF4, SF2, S2F2, S2F10 are also produced in small percentages. Not only these byproduct, impurities like air, moisture, and CO2 are also present in the gas, during production. All these byproducts and impurities are filtered at different stages of purification to get the pure and refined final product.
Chemical Properties
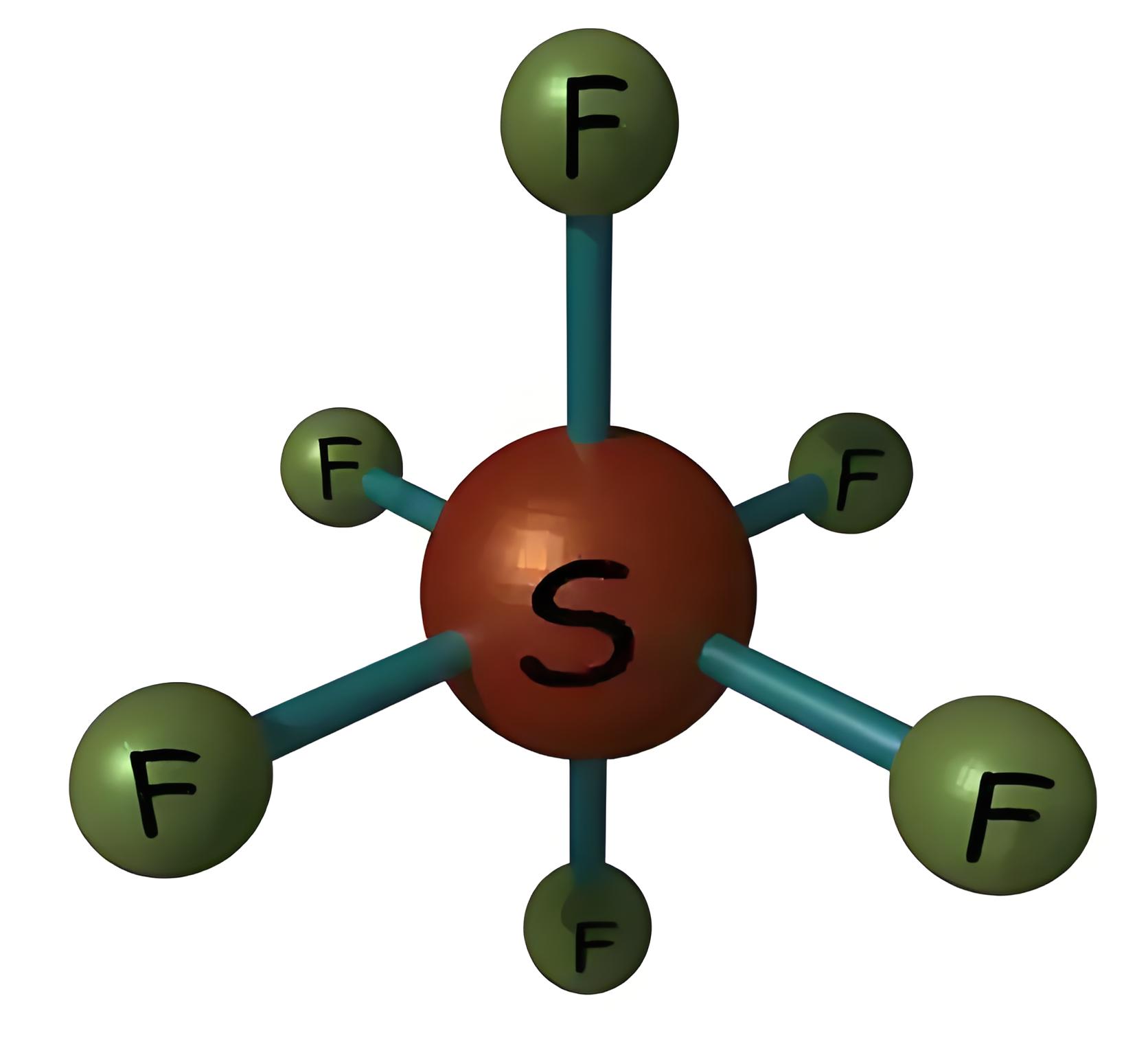
To understand the chemical properties of SF6 gas, we first look at its molecular structure. In an SF6 molecule, one sulfur atom is surrounded by six fluorine atoms.
The sulfur has atomic number of 16. Electronic configuration of sulphur atom is 2, 8, 6 i.e. 1Ssf6 gas2 2S2 2P6 3S2 3P4. The fluorine atom has atomic number 9. The electronic configuration of fluorine is 1S2 2S2 2P5. Each sulphur atom in SF6 molecule creates a covalent bond with 6 fluorine atoms. In this way, sulfur atom gets total 6 covalent bonds, i.e. 6 pairs of electrons at its outer shell, and each fluorine atom gets 8 electrons in its outer most shell.
NB: – Here we can observe that, in sulfur hexafluoride external shell of sulphur atom has 12 electrons instead of 8 electrons. That means here sulfur does not obey general octal rule of atomic structure which states that, a stable atom requires 8 electrons at its outermost shell. This is not an exceptional case. Some elements in the 3rd period and below can form a compound that exceeds 8 electrons in its outer most shell. The molecular structure of this gas is shown below,
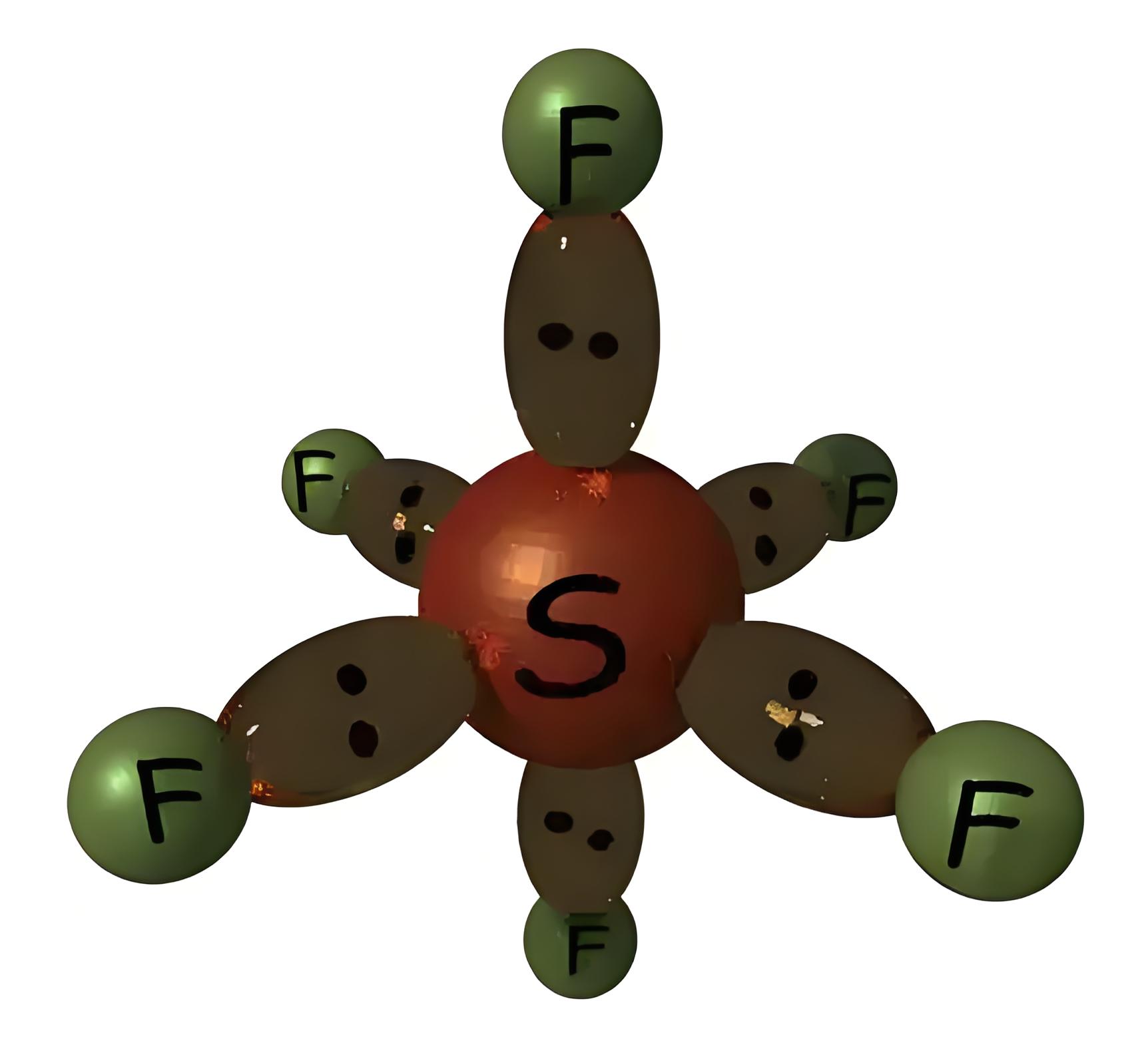
In this way, SF6 fully satisfies a stable structural condition. The effective radius of a sulfur hexafluoride molecule is 2.385 A. This electronic configuration and structure of this gas make SF6 extremely stable. The gas can be stable without any decomposition in its molecular structure up to 500oC. It is highly non-flammable. H2O and Cl cannot react with this gas. It also does not react with acid.
SF6 gas is one of the heaviest gases, with a density of 6.139 kg/m³ at 20°C and one atmospheric pressure, about five times denser than air. Its molecular weight is 146.06. The pressure-temperature variation is linear within the service range of -25 to +50°C. SF6 has a high volumetric specific heat, about 3.7 times that of air, giving it excellent cooling properties in electrical equipment. Despite its low thermal conductivity, SF6 is effective for cooling in circuit breakers because the gas absorbs and releases heat during molecular dissociation and reformation around an electric arc, quickly transferring heat from hot to cool areas.
SF6 gas is highly electronegative. Due to high electronegativity, it absorbs free electrons which are produced due to arcing between contacts of circuit breaker. Combination of free electrons with molecules produces heavy and big ions, which have very low mobility. Because of the absorption of free electrons and low mobility of ions SF6 has very excellent dielectric property. The dielectric strength of SF6 gas is about 2.5 times more than that of air.
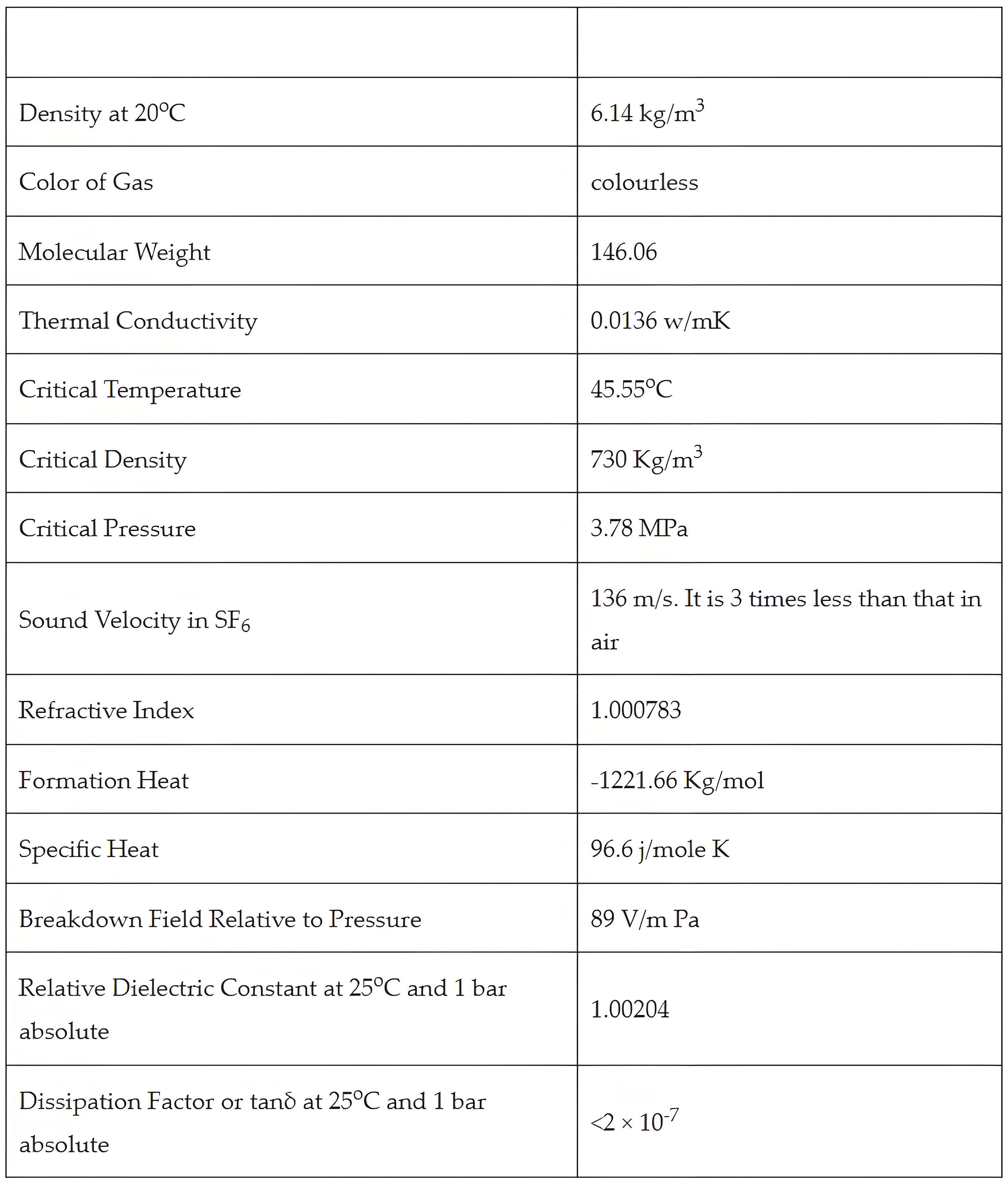
List of Sulphur Hexafluoride Gas Properties
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.













