What are Valence Electrons and Electrical Conductivity ?
What are Valence Electrons and Electrical Conductivity ?
Valence Electrons Definition
An atom is made up of a nucleus containing protons and neutrons, with electrons in shells around it. The nucleus is positively charged, and the electrons are negatively charged. Atoms are electrically neutral because they have equal numbers of protons and electrons.
Electrons in an atom are arranged in shells based on their energy levels. The closest shell to the nucleus has the lowest energy, while the farthest shell has the highest energy. Each shell has a maximum capacity for electrons: the first shell holds up to 2, the second up to 8, and so on.
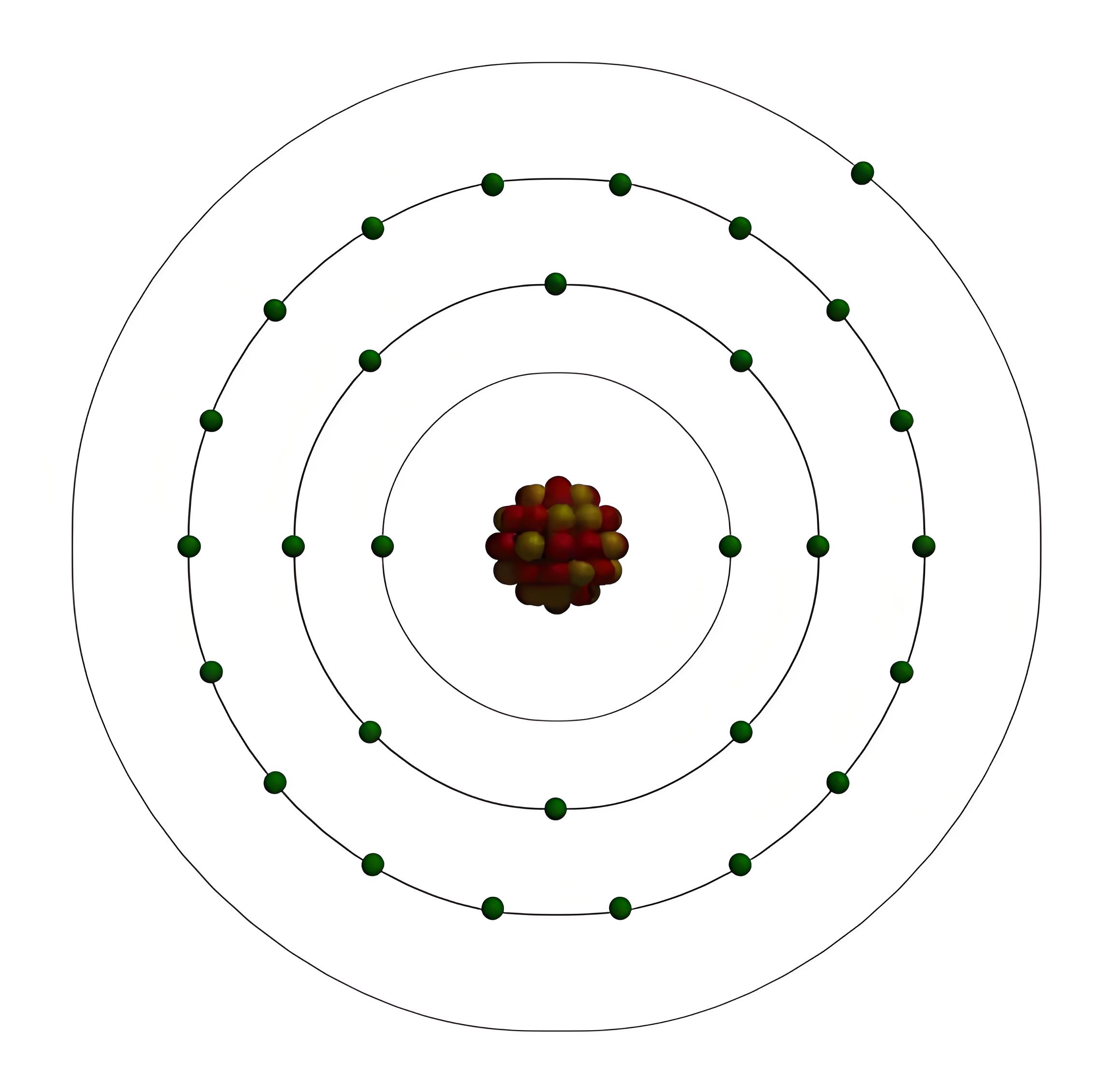
Valence electrons are the electrons in the outermost shell of atoms. They participate in chemical bonding and can be influenced by electric fields or magnetic fields. The number of valence electrons varies from 1 to 8, depending on the element.
Valence electrons are crucial in determining an element’s physical, chemical, and electrical properties. Elements with similar valence electrons usually have similar reactivity and bonding types. Different numbers of valence electrons result in varying electrical conductivities and material types.
Electrical Conductivity
Electrical conductivity measures how well a material allows electric current to flow through it. Electric current consists of moving electric charges, usually carried by free electrons or ions. High conductivity materials easily conduct current, while low conductivity materials resist it.
The electrical conductivity of a material depends on several factors, such as its temperature, structure, composition, and purity. However, one of the most important factors is the number and behavior of free electrons in the material.
Free electrons are valence electrons that are not tightly bound to their parent atoms and can move freely within the material. These are the electrons that can respond to an applied electric field or potential difference and drift toward one direction, creating an electric current.
The number and behavior of free electrons in a material are determined by the number of valence electrons in its constituent atoms. Generally, materials with fewer valence electrons tend to have more free electrons, while materials with more valence electrons tend to have fewer free electrons.
Based on their electrical conductivity and their number of valence electrons, materials can be classified into three main groups: conductors, semiconductors, and insulators.
Conductors
Conductors are materials that have high electrical conductivity because they have many free electrons that can easily carry an electric current. Conductors usually have one, two, or three valence electrons in their atoms. These valence electrons have high energy levels and are loosely attached to their parent atoms. They can easily detach from their atoms or move within the material when an electric field or potential difference is applied.
Most metals are good conductors of electricity because they have few valence electrons in their atoms. For example, copper has one valence electron, magnesium has two valence electrons, and aluminum has three valence electrons. These metals have many free electrons in their crystal structure that can move freely when an electric field is applied.
Some nonmetals can also act as conductors under certain conditions. For example, graphite (a form of carbon) has four valence electrons in its atoms, but only three of them are used for bonding with other carbon atoms in a hexagonal lattice. The fourth valence electron is free to move along the lattice when an electric field is applied.
Semiconductors
Semiconductors are materials that have moderate electrical conductivity because they have few free electrons that can carry an electric current under certain conditions. Semiconductors are materials that have four valence electrons in their atoms, such as carbon, silicon, and germanium. These valence electrons are used for bonding with other atoms in a regular lattice structure. However, at room temperature, some of these valence electrons can gain enough energy to break free from their bonds and become free electrons. These free electrons can then carry an electric current when an electric field is applied.
However, the number of free electrons in a pure semiconductor is very low, and the electrical conductivity is very poor. Therefore, semiconductors are often doped with impurity atoms that have either more or less valence electrons than the host atoms. This creates an excess or a deficiency of free electrons in the semiconductor, which increases its electrical conductivity.
There are two types of doping: n-type and p-type. In n-type doping, impurity atoms with five valence electrons, such as phosphorus or arsenic, are added to the semiconductor. These atoms donate one extra valence electron to the semiconductor, creating a negative charge carrier called an electron. In p-type doping, impurity atoms with three valence electrons, such as boron or gallium, are added to the semiconductor. These atoms accept one valence electron from the semiconductor, creating a positive charge carrier called a hole.
Semiconductors are widely used in various electronic devices, such as transistors, diodes, solar cells, light-emitting diodes (LEDs), lasers, and integrated circuits. These devices exploit the unique properties of semiconductors, such as their ability to switch between conducting and insulating states, their sensitivity to light and temperature, and their compatibility with other materials.
Insulators
Insulators are materials that have low electrical conductivity because they have very few or no free electrons that can carry an electric current. Insulators usually have five or more valence electrons in their atoms. These valence electrons are strongly bound to their parent atoms and require a lot of energy to be detached or excited. Therefore, insulators do not respond to an applied electric field or potential difference and resist or block the flow of electric current.
Most nonmetals are good insulators of electricity because they have many valence electrons in their atoms. For example, nitrogen has five valence electrons, sulfur has six valence electrons, and neon has eight valence electrons. These elements have no free electrons in their structure and do not allow electric current to flow through them.
Some materials can also act as insulators under certain conditions. For example, glass and rubber are good insulators at room temperature but can become conductors at high temperatures when some of their valence electrons gain enough energy to become free electrons.
Insulators are mainly used for preventing electric current from flowing where it is not wanted or needed. For example, insulators are used to coat wires and cables to protect them from short circuits and electric shocks. Insulators are also used to separate different parts of an electronic device or circuit to prevent unwanted interactions or interference.
Conclusion
Valence electrons are the electrons in the outermost shell of an atom that can participate in chemical bonding and electrical current. The number and arrangement of valence electrons determine many physical, chemical, and electrical properties of an element.
Electrical conductivity is a measure of how well a material can allow an electric current to flow through it. Electrical conductivity depends on several factors, such as the number and behavior of free electrons in the material.
Based on their electrical conductivity and their number of valence electrons, materials can be classified into three main groups: conductors, semiconductors, and insulators.
Conductors have high electrical conductivity because they have many free electrons that can easily carry an electric current. Conductors usually have one, two, or three valence electrons in their atoms.
Semiconductors have moderate electrical conductivity because they have few free electrons that can carry an electric current under certain conditions. Semiconductors usually have four valence electrons in their atoms.
Insulators have low electrical conductivity because they have very few or no free electrons that can carry an electric current. Insulators usually have five or more valence electrons in their atoms.
These materials have different applications in various electronic devices, such as transistors, diodes, solar cells, LEDs, lasers, and integrated circuits. These devices exploit the unique properties of these materials, such as their ability to switch between conducting and insulating states, their sensitivity to light and temperature, and their compatibility with other materials.
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.