What is a Voltaic Cell?
What is a Voltaic Cell?
Simple Voltaic Cell Definition
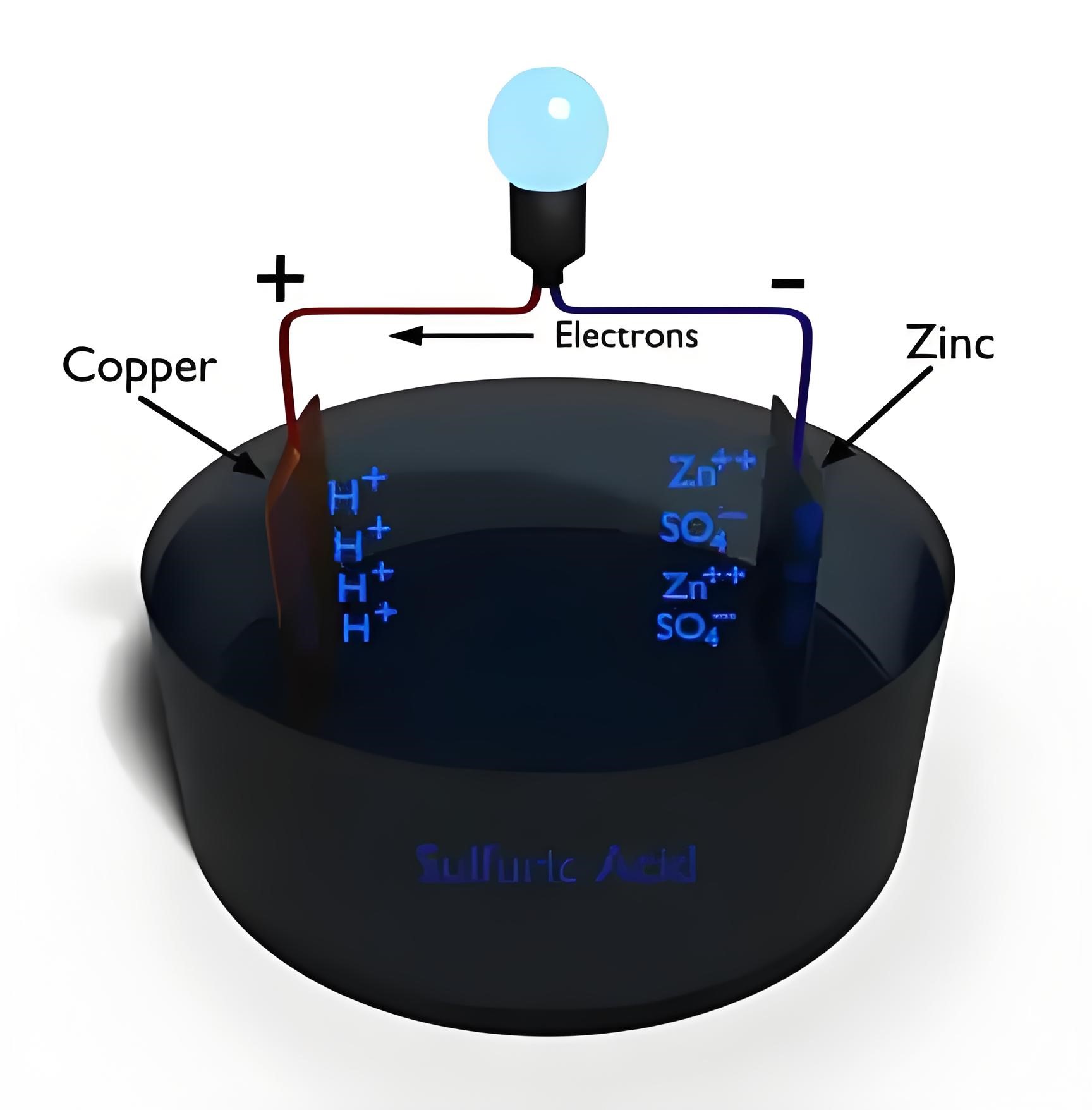
A simple voltaic cell is made by immersing zinc and copper plates in a diluted sulfuric acid solution, generating electricity.
Working Principle
The cell works because dissimilar metals in an electrolyte create a potential difference, causing electron flow.
Electron Movement
Electrons move from the zinc plate to the copper plate through an external circuit, generating current.
Polarization
Hydrogen buildup on the copper plate reduces current by increasing resistance, known as polarization.
Local Action
Impurities in zinc cause unwanted reactions that waste zinc, even when the cell is not producing current.
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.