What is loniaztion Energy?
What is loniaztion Energy?
Ionization Energy definition
Ionization Energy is the energy required for gaseous atoms in the ground state to lose electrons and become gaseous cations (i.e., ionization), which must overcome the gravitational pull of the nuclear charge on the electrons.
Bohr model interpretation
The Bohr model explains ionization energy by showing that electrons move around the nucleus at a fixed energy level.
Continuous ionization Energy
The first ionization Energy is always smaller than the second because it becomes more difficult to remove more electrons due to the increased attraction.
Electrical conductivity and ionization Energy of metals
Metals with low ionization Energy, such as silver and copper, have high conductivity because their electrons move easily.
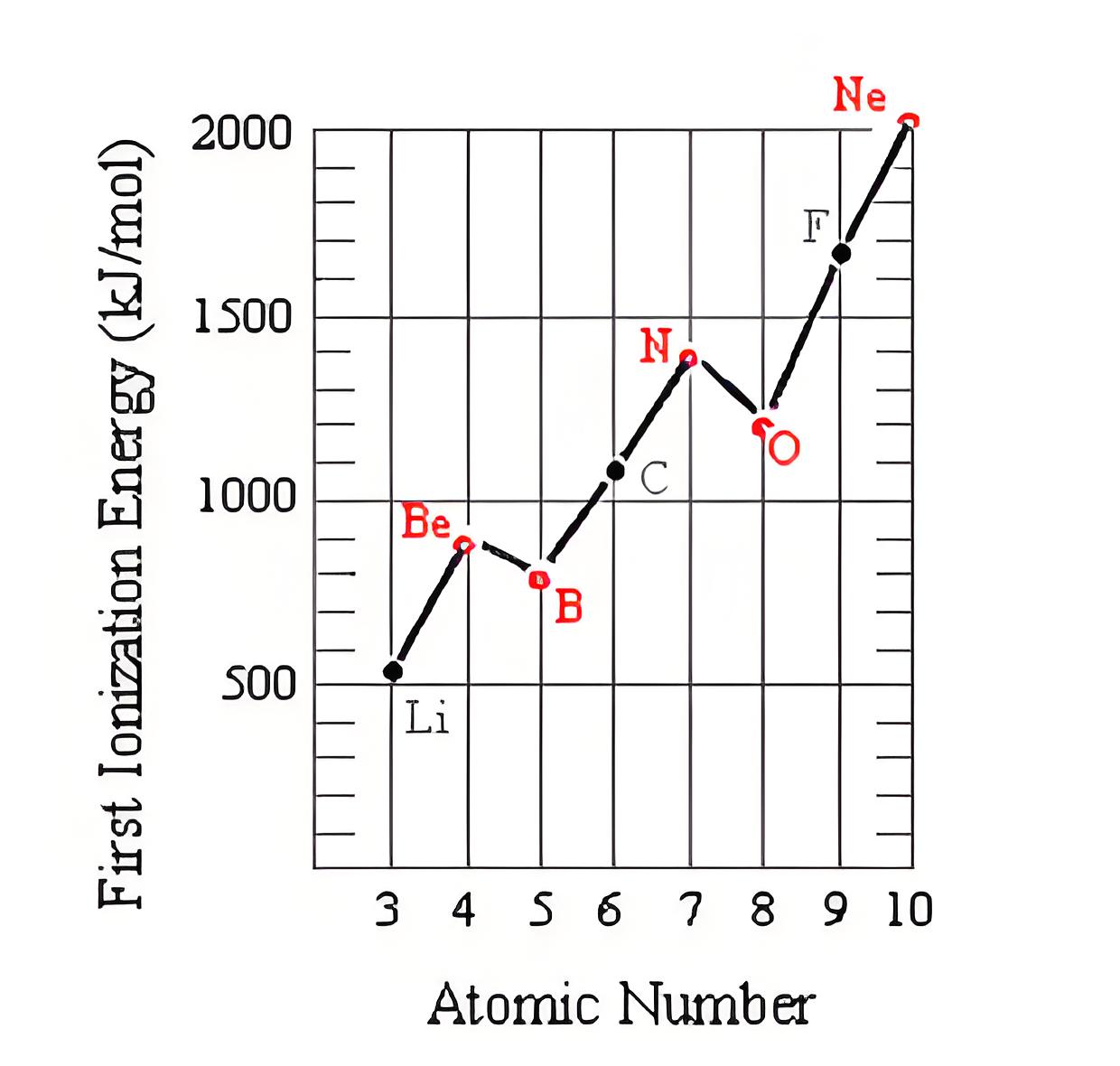
Factors affecting ionization Energy
Factors include atomic size
Shielding effect
Nuclear charge and electron configuration
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.