What is the Ionization Process?
What is the Ionization Process?
Ionization Definition
Ionization is a fundamental concept in chemistry and physics that describes the transformation of electrically neutral atoms or molecules into electrically charged ones.
Ionization Process
The ionization process involves the transfer of electrons between atoms or molecules.
Sodium Chloride Example
Both Na and Cl atoms are unstable or chemically active. When they come close to each other, they undergo a chemical reaction that involves the exchange of electrons. The Na atom loses its valence electron and becomes a positively charged ion (Na+), while the Cl atom gains an electron and becomes a negatively charged ion (Cl-). This process is called ionization.
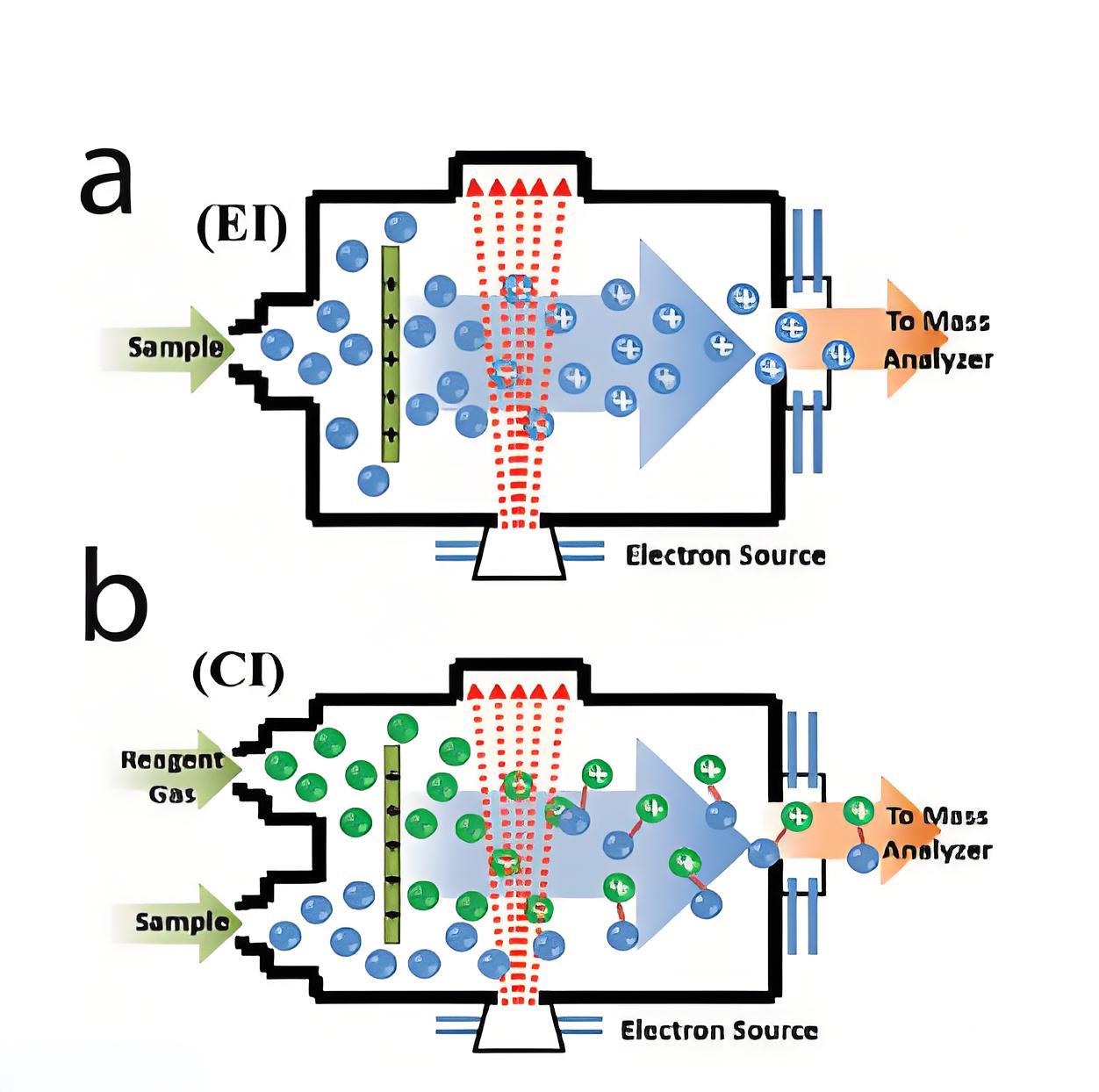
Ionization lnfluence Factor
Ionization Energy
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.