What is Ionic Polarization ?
What is Ionic Polarization ?
Ionic Polarization Definition
Ionic polarization is the shift of negative ions toward the positive side and positive ions toward the negative side in a molecule when an external electric field is applied.
Formation of Sodium Chloride
odium chloride (NaCl) forms through an ionic bond between sodium and chlorine, resulting in positive and negative ions that create a dipole moment
Permanent Dipole Moments
Some molecules have a permanent dipole moment due to their asymmetrical structure, present even without an external electric field.
External Electric Field Effect
Applying an external electric field causes ions in a molecule to shift, leading to ionic polarization.
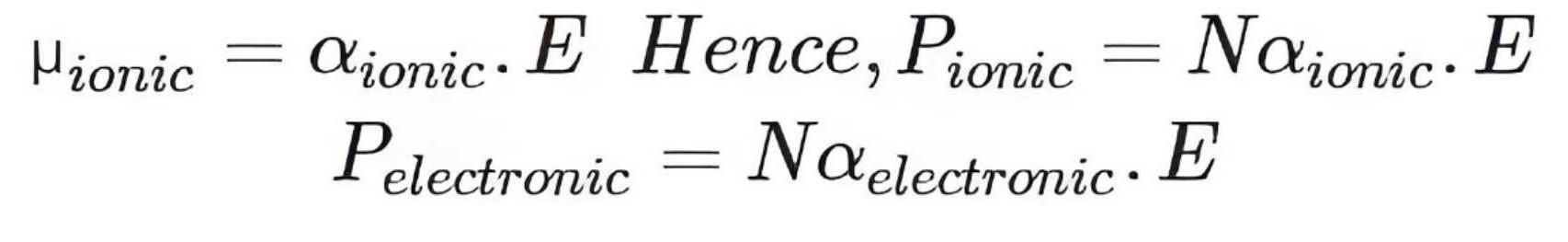
Types of Polarization
In ionic compounds, both ionic and electronic polarization occur when an electric field is applied, with the total polarization being the sum of both.
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.