How to Discharge a Bettery ?
How to Discharge a Bettery ?
Charging and Discharging Definition
Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions.
Oxidation Reaction
Oxidation happens at the anode, where the material loses electrons.
Reduction Reaction
Reduction happens at the cathode, where the material gains electrons.
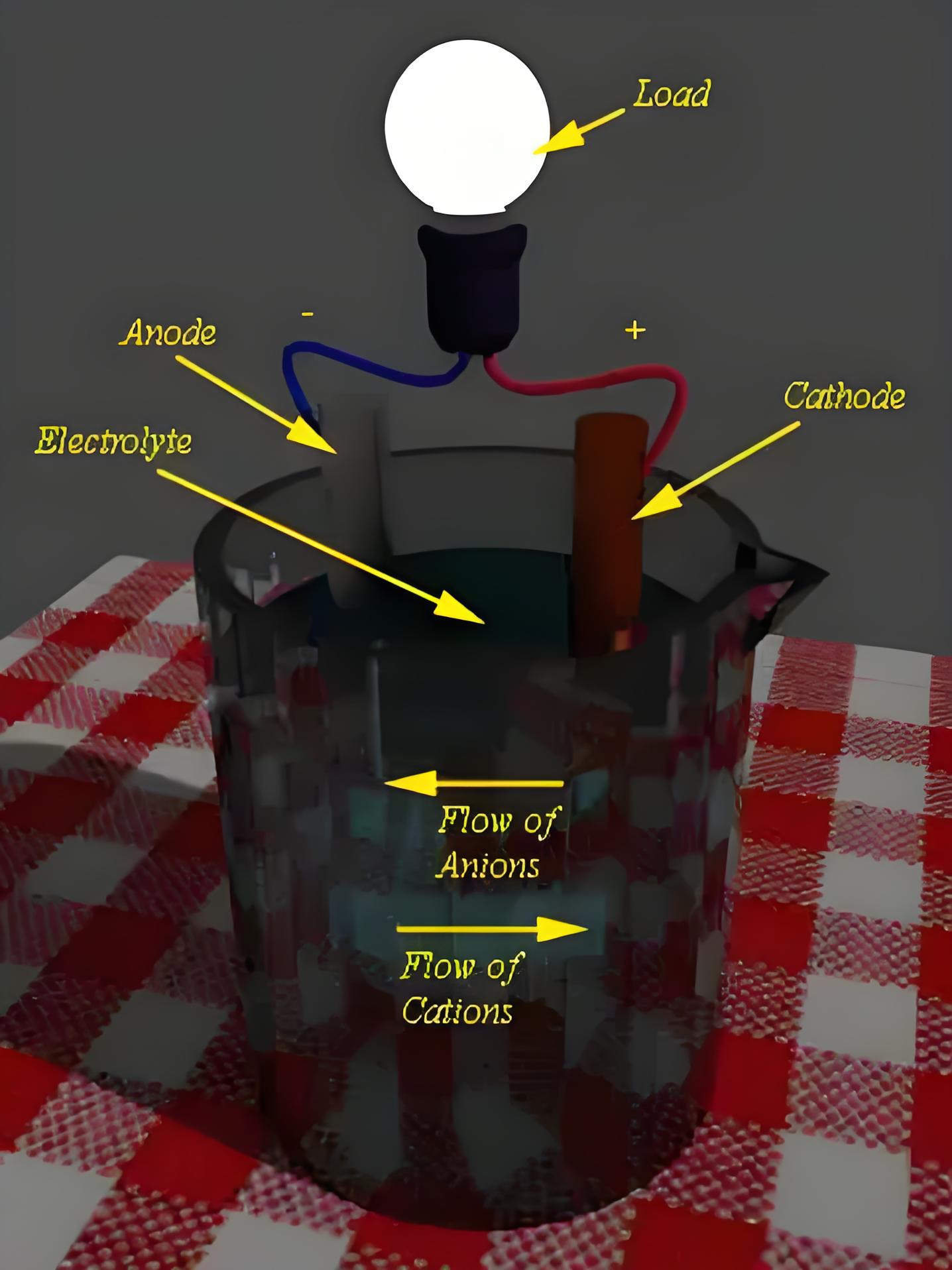
Discharging of Battery
In a battery there are two electrodes immersed in an electrolyte. When an external load is connected to these two electrodes, oxidation reaction starts occurring in one electrode and at the same time reduction occurs in other electrode.
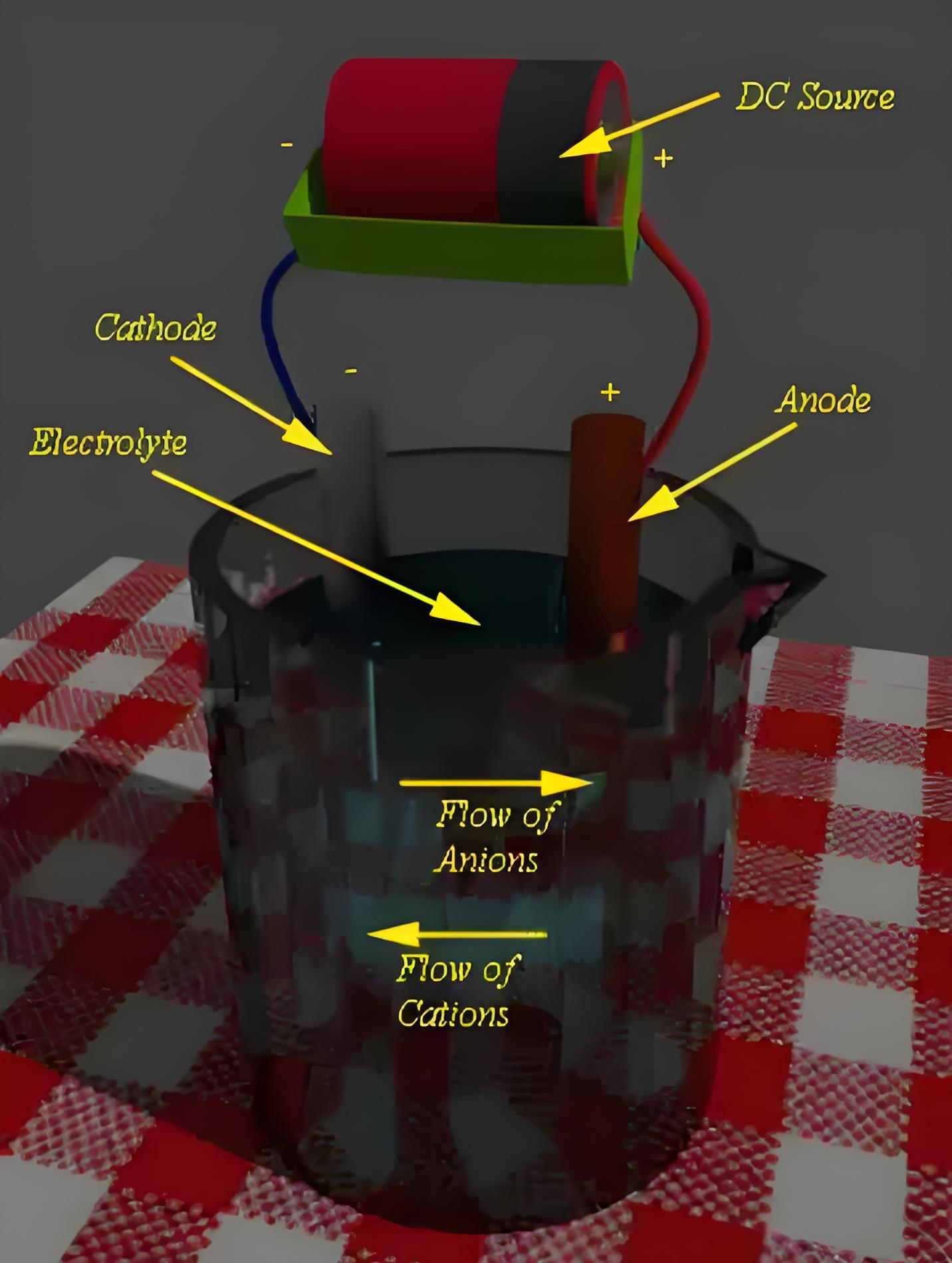
Charging of Battery
The external DC source injects electrons into the anode during charging. Here, reduction takes place at the anode instead of the cathode. This reaction allows the anode material to regain electrons, returning to its original state before the battery discharged.
Electron Flow in Discharge
During discharging, electrons flow from the anode to the cathode through an external circuit.
Role of External DC Source in Charging
An external DC source is used in charging to reverse the discharging reactions, restoring the battery to its charged state.
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.













