What is Transformer Acidity Test?
What is Transformer Acidity Test?
Acidity Test Definition
The acidity test of transformer oil measures the amount of potassium hydroxide (KOH) needed to neutralize the acid in the oil.
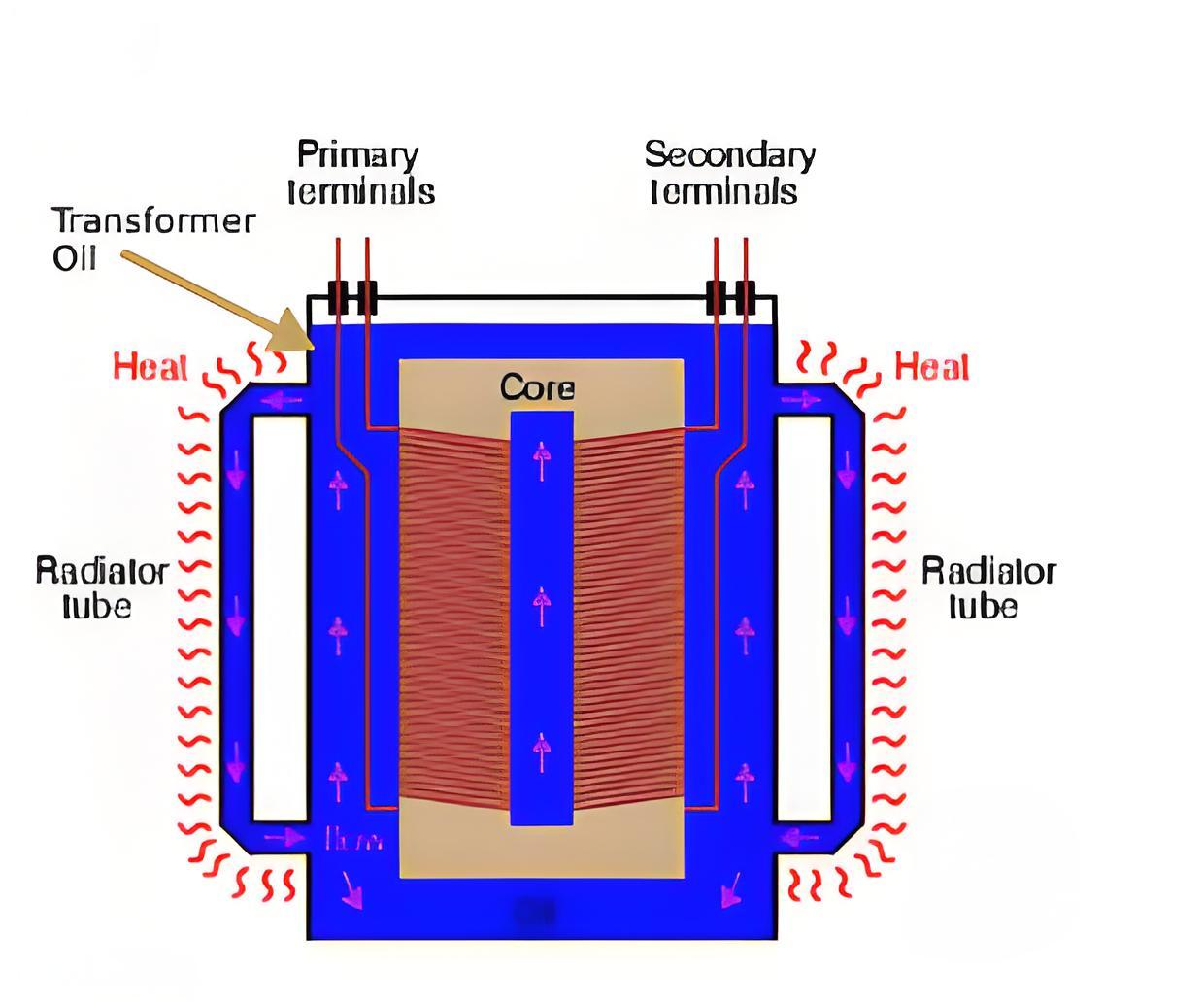
Causes of Acidity
Acidity occurs due to oxidation, especially when oil contacts air, and is accelerated by heat and metals like iron and copper.
Effects of Acidity
Higher acidity lowers oil resistivity, increases the dissipation factor, and can harm transformer insulation.
Acidity Test Kit Components
We can determine the acidity of transformer insulating oil, by a simple portable acidity test kit. It consists of one polythene bottle of rectified spirit (ethyl alcohol), one polythene bottle of sodium carbonate solution and one bottle of universal indicator (liquid). It also consists of clear and transparent test tubes and volumetrically scaled syringes.

Principle of Acidity Test of Insulating Oil
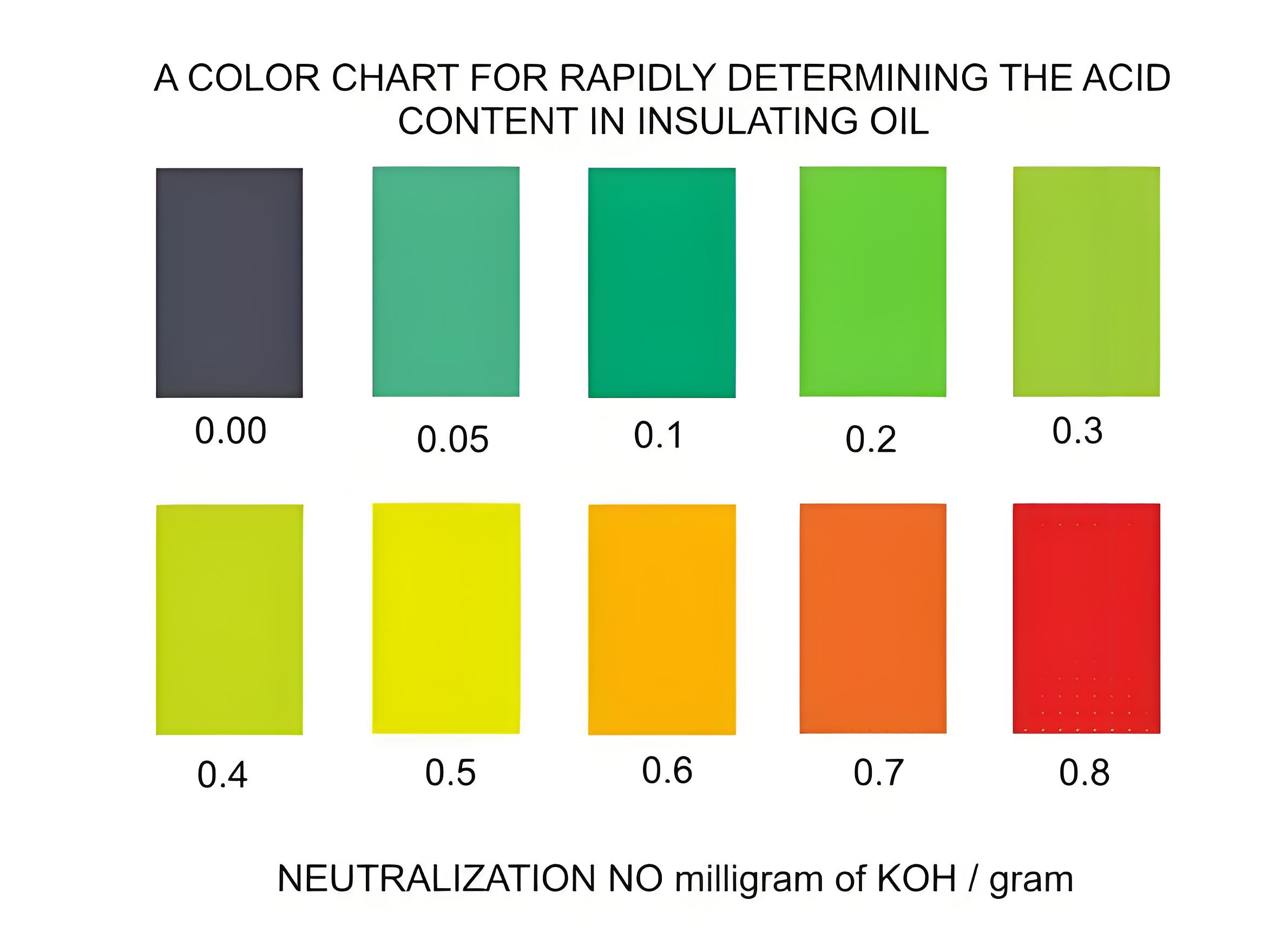
Adding alkali to the oil changes its acidity based on the acid amount present. If the alkali added equals the acid present, the oil’s pH will be 7 (neutral). More alkali makes the oil alkaline (pH 8-14), while less makes it acidic (pH 0-6). The universal indicator shows different colors for different pH levels, allowing us to visually determine the oil’s acidity.
Acidity of Insulating Oil Measure
The acidity of insulating oil is measured by the amount of KOH (in milligrams) needed to neutralize the acid in a specific amount of oil (in grams). For example, if the oil has an acidity of 0.3 mg KOH/g, it means 0.3 milligrams of KOH is needed to neutralize 1 gram of the oil.
Testing Procedure
The procedure involves adding specific amounts of rectified spirit, sodium carbonate, and a universal indicator to the oil and observing the color change to determine acidity.
The Electricity Encyclopedia is dedicated to accelerating the dissemination and application of electricity knowledge and adding impetus to the development and innovation of the electricity industry.